Carbon : An important element – Solutions
1. Select the proper option and complete the statements
(single, all, double, ionic, carbon, give and take, hydrogen, multiple, share, most, covalent)
a. A carbon atom forms a ….……………. bond with other atoms. In this bond the two atoms ….electrons.
Answer: covalent, share
b. All the carbon bonds in a saturated hydrocarbon ….……………. electrons.
Answer: share
c. At least one carbon bond in an unsaturated hydrocarbon is ….…………… .
Answer: multiple
d. ….……………. is the essential element in all the organic compounds.
Answer: Carbon
e. The element hydrogen is present in ….. organic compound.
Answer: all
2. Answer the following questions
a. Why are carbon and its compounds used as fuels?
Answer:
(i) The name ‘carbon’ is derived from Latin word ‘carbo’meaning coal. In the earth’s crust, carbon is present to an extent of approximately 0.27% in the form of carbonate, coal and petroleum. One of the non-crystalline and amorphous form of carbon is coal. Coal is a fossil fuel.
(ii) Peat, lignite, bituminous and anthracite are the four types of coal in the increasing order of their carbon content and heat produced respectively. Charcoal and coke are the other amorphous forms of carbon used as fuel.
(iii) Compounds of carbon such as hydrocarbons consist of carbon and hydrogen and they are easily combustible. For example, methane (CH4) which occurs in natural gas is highly inflammable. It bums by reacting with oxygen to give a bluish flame. In this reaction, 213 Kcal/mol of heat is given out. Methane bums completely.
Chemical reaction:
CH4 + 2O2 → CO2 + 2H2O + Heat
(iv) Thus when hydrocarbons are burnt in air, large amount of heat is evolved with formation of carbon dioxide (CO2) and water (H2O). Due to evolution of heat on combustion, carbon and its compounds are used as fuels.
b. In which compound forms does carbon occur?
Answer:
Carbon in its combined state exists as various compounds such as:
- Carbon dioxide and in the form of carbonates such as calcium carbonate, marble, calamine (ZnCO3).
- Fossil fuel – coal, petroleum, natural gas.
- Carbonaceous nutrients – carbohydrates, proteins, fats.
- Natural fibres – cotton, wool, silk.
- Hydrocarbons – compound of carbon and hydrogen.
c. Write the uses of the diamond.
Answer:
Uses of diamonds are:
- Diamonds are used in glass cutting and rock drilling machines.
- Diamonds are used in ornaments.
- Diamond knives are used in the eye surgery.
- Diamond dust is used for polishing other diamonds.
- Diamond is used to make windows giving protection from radiation in space and in artificial satellites.
3. Explain the difference:
a. Diamond and graphite.
Answer:
| Diamond | Graphite |
| (i) Diamond is a brilliant, hard and crystalline allotrope of carbon. | (i) Graphite is a black, soft, brittle and slippery crystalline allotrope of carbon. |
| (ii) In diamonds, every carbon atom is bonded to four neighbouring atoms by covalent bonds forming tetragonal three dimensional structure which makes it very hard. | (ii) In graphite, every carbon atom is bonded to three other carbon atoms by covalent bonds in such a way that a hexagonal layered structure is formed. A graphite crystal is made of many such layers of carbon atoms. These layers slip over each other on applying pressure. |
| (iii) Density of diamond is 3.5 g/cm3. | (iii) Density of graphite is 1.9 to 2.3 g/cm3. |
| (iv) Diamond is a bad conductor of electricity as it does not have free electrons. | (iv) Inside each layer of graphite, free electrons move continuously within the entire layer. Hence, graphite is a good conductor of electricity. |
b. Crystalline and non-crystalline forms of carbon.
Answer:
| Crystalline forms of carbon | Non-crystalline forms of carbon |
| (i) A crystalline form has a regular and definite arrangement of atoms. (ii) They have high melting points and boiling points. (iii) A crystalline form has a definite geometrical shape, sharp edges and plane surfaces. (iv) Diamond, graphite and fullerene are different crystalline forms of carbon. | (i) A non-crystalline form does not have a regular and definite arrangement of atoms. (ii) They have low melting points and boiling points. (iii) They are amorphous, hence, they do not have definite geometrical shape. (iv) Coal, charcoal and coke are different noncrystalline/amorphous forms of carbon. |
4. Write scientific reasons
a. Graphite is a conductor of electricity.
Answer:
- In graphite, each carbon is bonded to three other carbon atoms in such a way that a hexagonal layered structure is formed.
- Due to this structure, graphite has free electrons available.
- These free electrons move continuously within the entire layer.
- Hence, graphite is a good conductor of electricity.
b. Graphite is not used in ornaments.
Answer:
- Graphite is a black, soft, brittle and dull form of carbon.
- It is neither malleable nor ductile.
- These properties of graphite make it unsuitable for making of ornaments.
- Hence, graphite is not used for making ornaments.
c. Limewater turns milky when CO2 is passed through it.
Answer:
- Limewater traditionally means a weak solution of the alkali calcium hydroxide Ca(OH)2.
- When CO2 is passed through limewater, it reacts with calcium hydroxide to form insoluble particulates (precipitate) of Calcium carbonate (CaCO3).
- Calcium carbonate is weak basic salt and this gives a milky white precipitate.
- Hence, lime water turns milky when CO2 gas is passed through it.
d. Biogas is an eco-friendly fuel.
Answer:
- Biogas is formed by the decomposition of animal dung, dry leaves, wet garbage in a biogas plant.
- This produces methane gas also called biogas.
- Biogas is a very cheap fuel option which meets the demand for cooking gas.
- Biogas is eco-friendly as it contains about 55% to 60% of methane and rest is carbon dioxide, hence, on combustion it does not produce harmful gases which cause pollution.
- Biogas is a fuel which is convenient to use and in addition to this it produces a very good manure as a side product of the process.
- Hence, biogas is an eco-friendly fuel.
5. Explain the following.
a. Diamond, graphite and fullerenes are crystalline forms of carbon.
Answer:
- Diamond, graphite, and fullerenes are crystalline forms of carbon because their carbon atoms are arranged in a regular, repeating pattern, a key feature of crystalline structures. Each has a unique arrangement, giving them distinct properties:
- Diamond: Each carbon atom is bonded to four others in a strong, 3D tetrahedral structure. This orderly arrangement makes diamond very hard, with a high melting point (3500°C), and a non-conductor of electricity. Its clear, geometric shape shows its crystalline nature.
Graphite: Carbon atoms form hexagonal layers, with each atom bonded to three others in a layer. These layers are weakly connected, making graphite soft and slippery. Its regular, layered structure allows it to conduct electricity, and it’s crystalline due to this orderly arrangement.
- Fullerenes: In fullerenes like C60, carbon atoms are arranged in a ball-like structure with pentagonal and hexagonal rings. This fixed, repeating pattern makes them crystalline. They can dissolve in some liquids and have special properties.
b. Methane is called marsh gas.
Answer:
- Methane is formed by the decomposition of plant and animal matter in swamps or marshy areas.
- As methane gas bubbles out from marshy area, it is called as marsh gas.
c. Petrol, diesel, coal are fossil fuels.
Answer:
(i) A fossil fuel is a fuel formed by natural processes, such as anaerobic decomposition of buried dead organisms. Fossil fuels contain high percentage of carbon. The word carbon is derived from the Latin word ‘Carbo’ meaning coal.
(ii) Coal is formed from the remains of trees and other vegetation. Approximately 350 million years ago, these remains were trapped on the bottom of swamps, accumulating layer after layer and creating a dense material called peat. As this peat was buried under more and more ground, the high temperature and pressure transformed it into coal.
(iii) Petrol and diesel are obtained from mineral oil. Mineral oil also called as crude oil or petroleum oil is formed from the remains of plants and animals that lived in the seas millions of years ago. This plant and animal matter has been drawn down and subjected to extremes of temperature and pressure over millions of years ago.
(iv) Mineral oil is commonly formed in rocks under the sea bed. The word petroleum is derived from Latin word ‘Rock oil’. Petrol and Diesel are obtained from mineral oil by the process called as fractional distillation.
Thus petrol, diesel and coal are fossil fuels.
d. Uses of various allotropes of carbon.
Answer:
Diamond: Used in cutting tools, jewelry, eye surgery knives, and spacecraft windows.
Graphite: Used as a lubricant, in pencils, carbon electrodes, paints, and arc lamps.
Fullerenes: Used as insulators, catalysts in water purification, and in superconductivity.
Amorphous Forms:
- Coal: Fuel for homes, factories, and power plants; used to make coke and coal gas.
- Charcoal: Purifies water and acts as fuel.
- Coke: Domestic fuel, reducing agent, and produces industrial gases.
e. Use of CO2 in fire extinguisher.
Answer:
- CO2 based fire extinguishers do not cause corrosion and are non-conductors of electricity.
- It is beyond their capacity to extinguish a big fire.
- Therefore these fire extinguishers are used to extinguish small scale fire of electrical and electronic equipments.
f. Practical uses of CO2.
Answer:
Practical uses of CO2 are:
- CO2 is used to make aerated drinks.
- CO2 obtained by chemical reaction or kept under pressure is used in fire extinguishers. Liquified CO2 is used to remove caffeine from coffee.
- Liquid CO2 is used as solvent in modem eco-friendly dry cleaning.
- Solid carbon dioxide is used in cold storage and to keep milk and milk products and frozen substances cool during transport. It is also used for getting special effects of a mist in dramas and movies.
6. Write two physical properties each.
a. Diamond
Answer:
Properties of diamond are:
- Brilliant and pure diamond is the hardest natural substance.
- The density of diamond is 3.5 g/cm3.
- The melting point of diamond is 3500 °C.
- When a diamond is heated at 800 °C in the presence of oxygen, CO2 is given away. In this process no other product besides CO2 is formed.
- Diamond does not dissolve in any solvent.
- Acids/bases have no effect on diamond.
- Diamond is a bad conductor of electricity as it does not have free electrons.
b. Charcoal
Answer:
- The charcoal that is made from animals is made from their bones, horns, etc.
- On the other hand, the charcoal made from plants is formed by combustion of wood in an insufficient supply of air.
c. Fullerene
Answer:
Properties of fullerenes are:
- Molecules of fullerenes are found in the form of buckyballs and buckytubes.
- There are 30 to 900 carbon atoms in one molecule of a fullerene.
- Fullerenes are soluble in organic solvents such as carbon disulphide, chlorobenzene.
7. Complete the following Chemical reactions.
1. ………………..+………………..→ CO + 2H2O + Heat
2. ………………..+………………..→ HCl + Cl + HCl
3. 2 NaOH + CO2 →………………..+………………..
Answer:
8. Write answers to the following in detail.
a. What are the different types of coal? What are their uses?
Answer:
Coal is a fossil fuel. It contains carbon, hydrogen and oxygen. It also contains nitrogen, phosphorus and sulphur. It occurs in the solid state. It is of four types.
- Peat: Formation of peat is the first step in the formation of coal. It contains a high proportion
of water and less than 60% of carbon. Therefore, not much heat can be obtained from peat. - Lignite: Peat was transformed into Lignite due to increased pressure and temperature inside the earth. It contains 60 to 70% of carbon.- Lignite is the second step of the formation of coal.
- Bituminous coal: Bituminous coal was formed as the third step of formation of coal. It contains 70 to 90% of carbon.
- Anthracite: Anthracite is known as the pure form of coal. This coal is hard and contains about 95% of carbon.
Uses of coal:
- Coal is used as fuel in factories and homes.
- Coal is used to obtain coke, coal gas and coal tar.
- Coal is used in thermal power plants for generation of electricity.
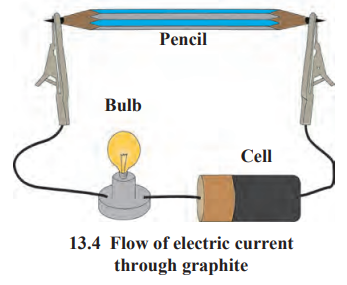
b. How will you prove experimentally that graphite is good conductor of electricity?
Answer:
Apparatus required: Lead pendi, electrical wires, battery/cell, small bulb, etc.
Step-I: Remove the lead from a pencil and assemble the apparatus as shown in the diagram.
Step-II:
- Start the electric current in the circuit, the moment the electric current is passed through the circuit, the bulb glows.
- This experiment proves that graphite is a good conductor of electricity as graphite has free electrons moving continuously within the entire layer and these free electrons conduct electricity in the lead of the pencil.
c. Explain the properties of carbon.
Answer:
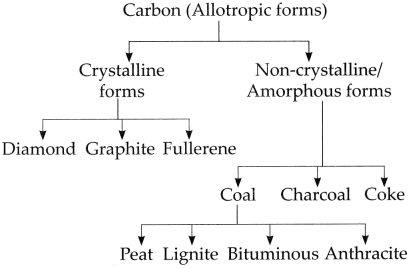
Allotropic nature of Carbon: Some elements occur in nature in more than one form. The chemical properties of these different forms are the same but their physical properties are different. This property of elements is called allotropy. Carbon shows the property of allotropy.
Carbon allotropes are of two types:
(A) Crystalline forms:
Carbon has three crystalline allotropes: Diamond, Graphite and Fullerene. Properties of crystalline forms of carbon are as follows:
- A crystalline form has a regular and definite arrangement of atoms.
- They are made up of only carbon atoms.
- They have high melting points and boiling points.
- A crystalline form has a definite geometrical shape, sharp edges and plane surfaces.
(B) Amorphous forms or non-crystalline forms: Coal, charcoal, coke are the non-crystalline forms of carbon.
Properties of non-crystalline forms of carbon are as follows:
- The arrangement of carbon atoms in this form is not regular.
- Apart from carbon atoms, they also contain hydrogen, oxygen, nitrogen, phosphorus and sulfur atoms.
- Compared to a crystalline form, they have low melting and boiling points.
- Most of them are used as fuels.
d. Classify carbon.
Answer:
Carbon is classified as follows:
9. How will you verify the properties of carbon dioxide?
Answer:
Properties of carbon dioxide can be verified in the following ways:
- When a burning candle is placed in a gas jar of carbon dioxide, it extinguishes indicating that carbon dioxide is a non-combustible gas and does not support combustion.
- When carbon dioxide gas is passed through lime water, it turns lime water milky due to the formation of insoluble calcium carbonate.
- Moist blue litmus turns red in a gas jar of carbon dioxide indicating, it is acidic in nature.
- Carbon dioxide is fairly soluble in water, it dissolves in water forming carbonic acid.

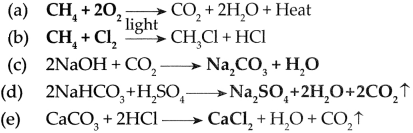
Leave a Reply