Measurement of Matter – Solutions
1. Give examples.
a. Positive radicals
Answer : H+, Na+, K+, Ag+, Cu+, Hg+
b. Basic radicals
Answer : Na+ – Sodium ion, K+ – Potassium ion, Ag+ – Silver ion
c. Composite radicals
Answer :
d. Metals with variable valency
Answer:
(a) Iron (Ferrum)
(i) Fe2+ – Ferrous [Iron – II]
(ii) Fe3+ – Ferric [Iron – III]
(b) Copper (Cuprum)
(i) Cu+ – Cuprous [Copper -1]
(ii) Cu2+ – Cupric [Copper – II]
(c) Mercury (Hydragyrum)
(i) Hg+ – Mercurous [Mercury -1]
(ii) Hg2+ – Mercuric [Mercury – II]
e. Bivalent acidic radicals
Answer:
S2- ,O2- ,Se2-
f. Trivalent basic radicals
Answer:
Al3+ – Aluminium, Cr3+ – Chromium, Fe3+ – Ferric.
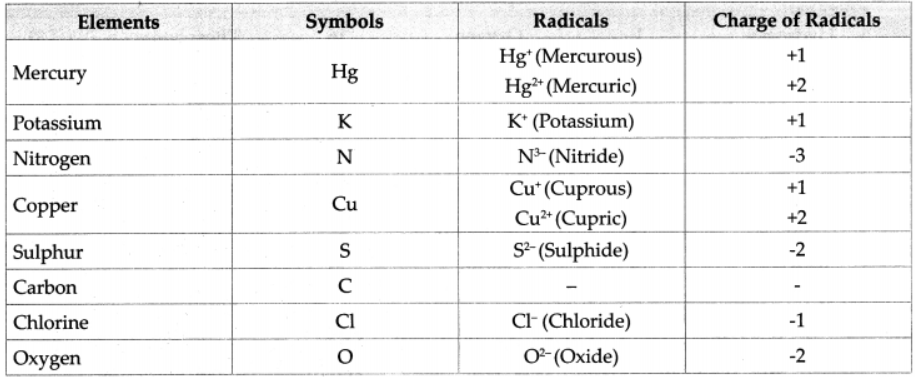
2. Write symbols of the following elements and the radicals obtained from them, and indicate the charge on the radicals.
Mercury, potassium, nitrogen, copper, sulphur, carbon, chlorine, oxygen
Answer :
3. Write the steps in deducing the chemical formulae of the following compounds.
Sodium sulphate, potassium nitrate, ferric phosphate, calcium oxide, aluminium hydroxide
Answer:
In order to write the chemical formulae of compounds, it is necessary to know the symbols and valency of various radicals.
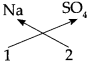
1. Sodium Sulphate:
Step – 1 : To write the symbols of the radicals (Basic radicals on the left and acidic radicals on the right)
Na2 SO4
Step – 2 : To write the valency below the respective radical.
Step – 3: To cross-multiply as shown by arrows the number of radicals.
Step – 4 : To write down the chemical formula of the compound.
Na2 SO4
(Sodium sulphate)
2. Potassium Nitrate:
The Symbols and Valencies of Potassium and Nitrate.
| Symbol | K | NO3 |
| Valency | 1 | 1 |
Cross-multiply the valencies
The chemical formula of the Potassium nitrate is KNO3.
Step 1: To write the symbols of the radicals (Basic radicals on the left and acidic radicals on the right) Fe PO4
Step 2: To write the valency below the respective radical.
Step 3: To cross-multiply as shown by arrows the number of radicals.
Step 4: To write down the chemical formula of the compound.
FePO4
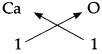
4. Calcium oxide:
The Symbols and Valencies of Calcium and Oxygen.
| Symbol | Ca | O |
| Valency | 2 | 2 |
Cross-multiply the valencies
The chemical formula of Calcium oxide is CaO.
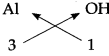
The Symbols and Valencies of Aluminium and Hydroxide.
| Symbol | Al | OH |
| Valency | 3 | 1 |
Cross-multiply the valencies
The chemical formula of Aluminium hydroxide is Al(OH)3.
4. Write answers to the following questions and explain your answers.
a. Explain how the element sodium is monovalent.
Answer:
- The number of protons or electrons (atomic number) in Sodium (Na) atom is 11. Therefore the electronic configuration of sodium atom is (2, 8,1).
- In chemical reaction, sodium atom has the capacity to give away le_ from its outermost orbit to form Na+ ion with stable electronic configuration (2, 8).
- As sodium atom gives away le- and a cation of sodium is formed, hence the valency of sodium is 1 and therefore, the element sodium is monovalent.
b. M is a bivalent metal. Write down the steps to find the chemical formulae of its compounds formed with the radicals, sulphate and phosphate.
Answer:
M is a bivalent metal. Following are the steps to find the chemical formulae of its compounds formed with the radicals, sulphate and phosphate:
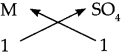
The symbols and valencies of M and sulphate:
| symbols | M | SO4 |
| valencies | 2 | 2 |
Cross-multiply the valencies
The chemical formula is MSO4.
The symbols and valencies of M and phosphate:
| symbols | M | PO4 |
| valencies | 2 | 3 |
Cross-multiply the valencies
The chemical formula is M3(PO4)2.
c. Explain the need for a reference atom for atomic mass. Give some information about two reference atoms.
Answer:
- The mass of an atom is concentrated in its nucleus and it is due to the protons (p) and neutrons (n) in it.
- Since an atom is very very tiny, it was not possible to measure atomic mass accurately. Therefore, the concept of relative mass of an atom was formed.
- To express relative mass of an atom, reference of atom is considered. The two reference atoms were as follows:
(a) Hydrogen (H) atom: The hydrogen atom is the lightest. The relative mass of a hydrogen atom is 1 which has only 1 proton in its nucleus. On this scale, the relative atomic mass of many elements comes out to be fractional. Therefore, carbon was selected as a reference atom.
(b) Carbon (C) atom: The carbon atom is selected as reference atom. In this scale, the relative mass of a carbon atom is accepted as 12.
- The relative atomic mass of 1 hydrogen (H) atom compared to the carbon (C) atom becomes
d. What is meant by Unified Atomic Mass.
Answer:
- During earlier time, relative mass of an atom was considered for measuring the mass of an atom directly. But since the founding of unified mass, relative mass is not accepted henceforth.
- Unified atomic mass is the unit of atomic mass called as Dalton.
- Its symbol is ‘u’. lu = 1.66053904 x 10-27 kg.
e. Explain with examples what is meant by a ‘mole’ of a substance.
Answer:
- A mole is that quantity of a substance whose mass in grams is equal in magnitude to the molecular mass of that substance in Daltons.
- For example: Atomic mass of oxygen atom (O) is 16u. Thus, the molecular mass of oxygen molecule (O2) is 16 x 2 = 32u. Therefore, 32 g of oxygen is 1 mole of oxygen.
5. Write the names of the following compounds and deduce their molecular masses.
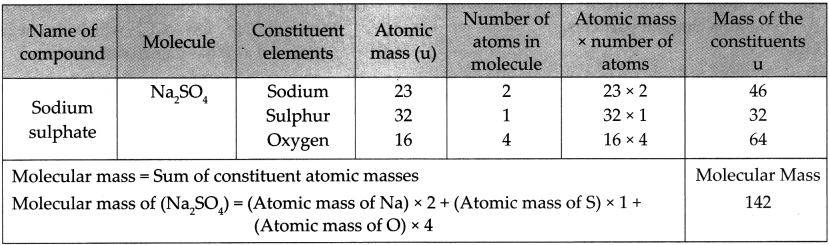
6. Two samples ‘m’ and ‘n’ of slaked lime were obtained from two different reactions. The details about their composition are as follows:
Mass of constituent oxygen : 2g
Mass of constituent calcium : 5g
‘sample n’ mass : 1.4g
Mass of constituent oxygen : 0.4g
Mass of constituent calcium : 1.0g
As it is mentioned already:
- Sample m Mass: 7g
- Mass of constituent oxygen: 2g
- Mass of constituent calcium: 5g
- Sample n Mass: 1.4g
- Mass of constituent oxygen: 0.4g
- Mass of constituent calcium: 1.0g
Out of all the laws of chemical combination, this is proved by the “Law of Constant Proportion”. The Law Of Constant Proportion states that “The proportion by weight of the constituent elements in various samples of the compound is fixed in ratio”.
For example: In sample m, the ratio of the proportion of elements (calcium: oxygen) is 5:2.
Ca:O = 5:2
For example: In sample n, the ratio of the proportion of elements (calcium: oxygen) is 1.0:0.4.
Ca:O = 1.0:0.4
= 10:4
= 5:2
On simplifying the ratio proportion by mass, we get the same values which verifies “The Law Of Constant Proportion”.
7. Deduce the number of molecules of the following compounds in the given quantities.
Number of moles in O2 = Mass of Oin gramsMolecular mass of O
=
= 1 mole
∴ 1 mole of O2 contains 6.022 × 1023 molecules.
∴ The number of molecules in 32 grams of oxygen is 6.022 × 1023.
90g water
Number of moles in water = Mass of water in gramsMolecular mass of water
=
= 5 moles
∴ 5 moles of H2O contains = 5 × 6.022 × 1023 molecules = 30.11 × 1023 molecules.
8.8g carbon dioxide
Number of moles in CO2 = Mass of COin gramsMolecular mass of CO
=
= 0.2 mole
∴ 0.2 moles of CO2 contains = 0.2 × 6.022 × 1023 molecules = 1.2044 × 1023 molecules.
∴ The number of molecules in 8.8 grams of carbon dioxide is 1.2044 × 1023.
The number of molecules in 90 grams of water is 3.01 × 1024.
7.1g chlorine
No. of moles in chlorine = Mass of chlorine in gramsMolecular mass of chlorine
=
= 0.1 mole
∴ 0.1 moles of Cl2 contains = 0.1 × 6.022 × 1023 molecules = 0.6022 × 1023 molecules.
∴ Number of molecules in 7.1 grams of chlorine is 6.022 × 1022.
8. If 0.2 mol of the following substances are required how many grams of those substances should be taken?
Sodium chloride
We know that,
Number of moles of a substance = Mass of the substance in gramsMolecular mass of the substance
Molar mass = sum of constituent atomic masses
Molar mass of NaCl = 23 + 35.5 = 58.5 g/mol
Number of moles of a substance = Mass of the substance in gramsMolecular mass of the substance
Let Mass of the substance in grams be x,
g
We need, 11.7 g of NaCl for obtaining 0.2 moles of NaCl.
magnesium oxide
Molar mass of MgO = 24 + 16 = 40 g/mol
Number of moles of a substance = Mass of the substance in gramsMolecular mass of the substance
Let Mass of the substance in grams be x,
x
g
we need, 8g of MgO to obtain 0.2 moles of MgO.
calcium carbonate
Molar mass of CaCO3 = 40 + 12 + 3(16) = 100 g/mol
Number of moles of a substance = Mass of the substance in gramsMolecular mass of the substance
Let Mass of the substance in grams be x,
x
g
we need, 20 g of CaCO3 for obtaining 0.2 moles of CaCO3.


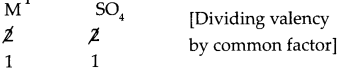
Leave a Reply