Solutions For All Chapters – Science Class 8
Nature of Matter: Elements, Compounds, and Mixtures
Keep the curiosity alive
1. Consider the following reaction where two substances, A and B, combine to form a product C:
A + B → C
Assume that A and B cannot be broken down into simpler substances by chemical reactions. Based on this information, which of the following statements is correct?
(i) A, B, and C are all compounds and only C has a fixed composition.
(ii) C is a compound, and A and B have a fixed composition.
(iii) A and B are compounds, and C has a fixed composition.
(iv) A and B are elements, C is a compound, and has a fixed composition.
Answer:- (iv) A and B are elements, C is a compound, and has a fixed composition.
2. Assertion: Air is a mixture.
Reason: A mixture is formed when two or more substances are mixed, without undergoing any chemical change.
(i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
(ii) Both Assertion and Reason are true, but Reason is not the correct explanation for Assertion.
(iii) Assertion is true, but Reason is false.
(iv) Assertion is false, but Reason is true.
Answer:- (i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
3. Water, a compound, has different properties compared to those of the elements oxygen and hydrogen from which it is formed. Justify this statement.
Answer: Water, a compound, has different properties compared to hydrogen and oxygen because it is formed by a chemical reaction creating a new substance. Hydrogen and oxygen are gases with no fixed shape or volume; hydrogen is flammable, and oxygen supports burning. Water is a liquid with a definite volume, does not burn, and extinguishes fires. These differences arise from the chemical bonding in water molecules (two hydrogen atoms and one oxygen atom), giving it unique physical and chemical properties unlike its constituent elements.
4. In which of the following cases are all the examples correctly matched? Give reasons in support of your answers.
(i) Elements – water, nitrogen, iron, air.
(ii) Uniform mixtures- minerals, seawater, bronze, air.
(iii) Pure substances- carbon dioxide, iron, oxygen, sugar.
(iv) Non-uniform mixtures – air, sand, brass, muddy water
Answer: (iii) Pure substances – carbon dioxide, iron, oxygen, sugar.
Justification: Carbon dioxide (CO₂) and sugar (sucrose) are compounds, made of multiple elements chemically combined with fixed composition. Iron and oxygen (O₂) are elements, also with fixed composition. All are pure substances, as they have uniform properties and cannot be broken down physically.
5. Iron reacts with moist air to form iron oxide, and magnesium burns in oxygen to form magnesium oxide. Classify all the substances involved in the above reactions as elements, compounds or mixtures, with justification.
Iron (Fe) + Oxygen (O2)+ Water (H2O) → Iron Oxide (Fe2O3)
Magnesium burns in oxygen to form magnesium oxide
Magnesium (Mg) + Oxygen (O2) → Magnesium Oxide (MgO)
- Iron: Element (pure metal, cannot be broken down).
- Moist air: Mixture (air gases plus water vapor, components retain properties).
- Iron oxide: Compound (iron and oxygen combined chemically).
- Magnesium: Element (pure metal).
- Oxygen: Element (pure gas).
- Magnesium oxide: Compound (magnesium and oxygen in fixed ratio).
Justification: Elements are simplest substances; compounds form from elements via chemical reactions with new properties; mixtures do not involve chemical bonding.
6. Classify the following as elements, compounds, or mixtures in Table 8.3.Carbon dioxide, sand, seawater, magnesium oxide, muddy water, aluminium, gold, oxygen, rust, iron sulfide, glucose, air, water, fruit juice, nitrogen, sodium chloride, sulfur,hydrogen, baking soda.
Answer:
| Elements | Compounds | Mixtures |
|---|---|---|
| Aluminium | Carbon dioxide | Sand |
| Gold | Magnesium oxide | Seawater |
| Oxygen | Rust | Muddy water |
| Nitrogen | Iron sulfide | Air |
| Sulfur | Glucose | Fruit juice |
| Hydrogen | Water | |
| Sodium chloride | ||
| Baking soda |
Pure substances:
- Aluminium
- Gold
- Oxygen
- Nitrogen
- Sulfur
- Hydrogen
- Carbon dioxide
- Magnesium oxide
- Rust
- Iron sulfide
- Glucose
- Water
- Sodium chloride
- Baking soda
7. What new substance is formed when a mixture of iron filings and sulfur powder is heated, and how is it different from the original mixture? Also, write the word equation for the reaction.
Answer:- When iron filings and sulfur powder are heated, they form a new substance called iron sulfide.
- Appearance: The mixture shows separate iron (black) and sulfur (yellow), but iron sulfide is a uniform black solid.
- Magnetic property: Iron in the mixture is attracted to a magnet, but iron sulfide is not.
- Chemical property: Iron reacts with dilute HCl to give hydrogen gas, while iron sulfide reacts with dilute HCl to give hydrogen sulfide gas (smells like rotten eggs).
Word Equation: Iron + Sulfur → Iron sulfide
8. Is it possible for a substance to be classified as both an element and a compound? Explain why or why not
Answer:- No, a substance cannot be classified as both an element and a compound. Elements are pure substances that cannot be broken down into simpler substances by chemical means, while compounds are formed when two or more different elements are chemically bonded together. The defining characteristic of a compound is that it is composed of multiple elements, whereas an element is a single type of atom. Therefore, a substance cannot be both a single type of atom and a combination of different types of atoms simultaneously.
9. How would our daily lives be changed if water were not a compound but a mixture of hydrogen and oxygen?
Answer:- If water were a mixture of hydrogen and oxygen instead of a compound, it would be dangerous to use. Hydrogen is flammable and oxygen supports burning, so the mixture could catch fire or explode. We would not be able to use it safely for drinking, cooking, bathing, or putting out fires.
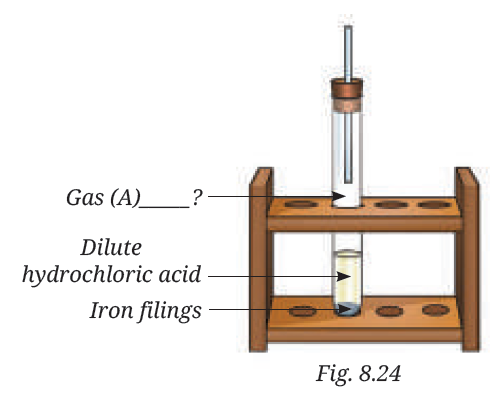
10. Analyse Fig. 8.24. Identify Gas A. Also, write the word equation of the chemical reaction.
Answer : Gas (A) is Hydrogen gas.
Word equation for the reaction:
Iron + Dilute Hydrochloric Acid → Iron Chloride + Hydrogen gas
11. Write the names of any two compounds made only from non-metals, and also mention two uses of each of them.
Answer : Two compounds made only from non-metals are:
1. Carbon Dioxide (CO₂) Uses:
- It is used in fire extinguishers to put out fires because it does not support combustion.
- It is used in carbonated drinks like soda to give them fizz.
2. Water (H₂O) Uses:
- It is essential for drinking and keeping our bodies hydrated.
- It is used for cooking food and cleaning purposes.
12. How can gold be classified as both a mineral and a metal?
Answer : Gold can be classified as both a mineral and a metal because:
1. As a mineral, gold is found in nature in a pure solid form. It is a native mineral, meaning it occurs naturally without being combined with other elements.
2. As a metal, gold is a shiny, malleable, and ductile element that conducts electricity and heat. It is used in making jewellery, coins, and electronic components.
Thus, gold is a naturally occurring mineral and also a useful metal due to its physical properties

Best answers ever
Very good next time , please you make short notes of every chapter
Good answer I liked it so much!
Very nice