Notes For All Chapters Science Class 9 CBSE
Introduction
When we see packets of milk, ghee, butter, salt, or juice labeled “pure,” we think pure means no adulteration.
→ Example: “Pure ghee” means no mixing of oil or impurities.
Scientifically, “pure” means:
→ A substance made up of only one kind of particles (same in chemical nature).
→ Example: Pure water contains only H₂O molecules — nothing else.
Therefore, milk, air, and juices are not pure scientifically — they are mixtures of several substances.
Mixture: Most matter around us exists as a mixture of two or more pure substances, e.g.:
- Sea water → salts + water
- Soil → sand, clay, minerals
- Air → oxygen + nitrogen + carbon dioxide
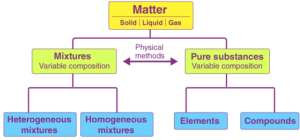
2.1 What is a Mixture?
Definition:
A mixture is a combination of two or more pure substances (elements or compounds) mixed in any proportion such that no chemical reaction occurs between them.
Characteristics of a Mixture:
It consists of two or more substances physically mixed.
The composition of a mixture is variable.
Components retain their original properties.
Components can be separated by physical methods like filtration, evaporation, etc.
Pure Substance vs Mixture:
| Pure Substance | Mixture |
|---|---|
| Made of one kind of particle. | Contains two or more substances. |
| Fixed composition. | Variable composition. |
| Cannot be separated by physical means. | Can be separated by physical means. |
| Examples: Salt, sugar, water. | Examples: Air, soil, milk. |
2.1.1 Types of Mixtures
Mixtures are mainly of two types:
1. Homogeneous Mixture
Definition: A mixture that has a uniform composition throughout.
The particles are not visible individually.
Examples:
- Salt dissolved in water
- Sugar dissolved in water
- Air (oxygen + nitrogen)
Characteristics:
- Composition is uniform.
- No visible boundaries between components.
- Also known as solutions.
- Can have variable concentration (one can be more concentrated or dilute).
2. Heterogeneous Mixture
Definition: A mixture with non-uniform composition, and components are visible separately.
Examples:
- Oil and water
- Sand and salt mixture
- Soil
Characteristics:
- Non-uniform composition.
- Different parts of mixture are physically distinct.
- Components can be separated easily by physical methods.
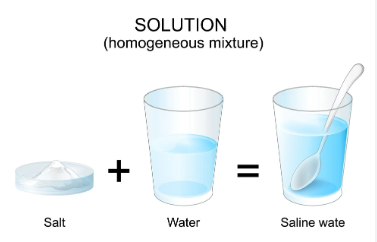
2.2 What is a Solution?
Definition:
A solution is a homogeneous mixture of two or more substances whose composition is uniform throughout.
Examples:
- Lemonade (water + lemon juice + sugar)
- Soda water (water + CO₂ gas)
- Air (mixture of gases)
- Alloys (solid solutions like brass and bronze)
Components of a Solution:
| Component | Description | Example |
|---|---|---|
| Solvent | Substance that dissolves the solute (usually in larger amount). | Water in sugar solution |
| Solute | Substance that gets dissolved in solvent (usually smaller amount). | Sugar or salt |
Examples of Solutions:
| Type of Solution | Solute | Solvent | Example |
|---|---|---|---|
| Solid in Liquid | Sugar | Water | Sugar solution |
| Solid in Liquid | Iodine | Alcohol | Tincture of iodine |
| Gas in Liquid | CO₂ | Water | Soda water |
| Gas in Gas | O₂ + CO₂ | N₂ | Air |
| Solid in Solid | Cu + Zn | – | Brass (alloy) |
Properties of a Solution:
- It is a homogeneous mixture.
- Particle size < 1 nm (10⁻⁹ m) — cannot be seen with naked eyes.
- Does not scatter light (path of beam not visible).
- Stable – particles do not settle down even on standing.
- Cannot be separated by filtration.
2.2.1 Concentration of a Solution
Definition: The amount of solute present in a given amount of solution.
Types of Solutions (by solute amount):
- Dilute Solution: Contains small amount of solute.
- Concentrated Solution: Contains more solute.
- Saturated Solution: Contains maximum amount of solute that can be dissolved at a given temperature.
- Unsaturated Solution: Can still dissolve more solute at that temperature.
Solubility:
- The amount of solute dissolved in 100 g of solvent at a given temperature to form a saturated solution.
- Solubility changes with temperature — generally increases with rise in temperature.
Methods to Express Concentration:
Mass by Mass %:
\(\text{Mass %} = \frac{\text{Mass of solute}}{\text{Mass of solution}} \times 100\)
Mass by Volume %:
\(\frac{\text{Mass of solute}}{\text{Volume of solution}} \times 100\)
Volume by Volume %:
\(\frac{\text{Volume of solute}}{\text{Volume of solution}} \times 100\)
Example:
40 g of salt dissolved in 320 g of water:
Total = 360 g solution
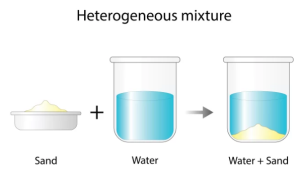
2.2.2 What is a Suspension?
Definition:
A suspension is a heterogeneous mixture in which insoluble particles remain suspended throughout the medium.
Examples: Chalk powder in water, sand in water, muddy water.
Properties of a Suspension:
- Heterogeneous mixture.
- Particles are visible to naked eye.
- Scatters light → path of beam visible.
- Unstable → particles settle down on standing.
- Can be filtered → residue remains on filter paper.
2.2.3 What is a Colloidal Solution (Colloid)?
Definition:
- A colloid (or colloidal solution) is a heterogeneous mixture in which particle size is intermediate between that of true solutions and suspensions.
- Examples: Milk, ink, blood, fog, butter, jelly.
Properties of a Colloid:
- Heterogeneous mixture (appears homogeneous to naked eye).
- Particles cannot be seen individually.
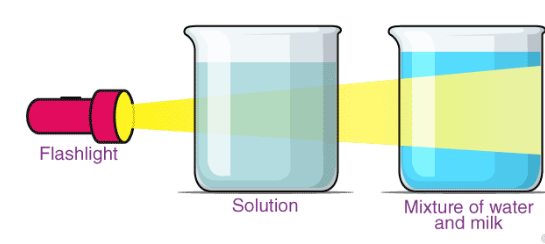
- Tyndall Effect: Scattering of light by colloidal particles.
- Stable: Particles do not settle down.
- Cannot be filtered by paper filter, but can be separated by centrifugation.
Tyndall Effect
Discovered by John Tyndall.
Definition: Scattering of light by colloidal particles making its path visible.
Examples:
- Sunlight through forest mist.
- Light through dusty air or milk.
- Not seen in true solutions.
Components of a Colloid:
| Component | Description |
|---|---|
| Dispersed Phase | Substance dispersed (like solute). |
| Dispersion Medium | The medium in which particles are dispersed (like solvent). |
Common Examples of Colloids:
| Dispersed Phase | Dispersion Medium | Type | Examples |
|---|---|---|---|
| Liquid | Gas | Aerosol | Fog, clouds, mist |
| Solid | Gas | Aerosol | Smoke, exhaust |
| Gas | Liquid | Foam | Shaving cream |
| Liquid | Liquid | Emulsion | Milk, face cream |
| Solid | Liquid | Sol | Mud, milk of magnesia |
| Gas | Solid | Foam | Sponge, pumice |
| Liquid | Solid | Gel | Jelly, butter |
| Solid | Solid | Solid Sol | Coloured glass, gemstones |
2.3 Physical and Chemical Changes
| Property | Physical Change | Chemical Change |
|---|---|---|
| Definition | Change in physical properties without changing composition. | Formation of new substances with new properties. |
| Reversibility | Mostly reversible. | Irreversible. |
| Examples | Melting, boiling, dissolving salt, cutting paper. | Burning, rusting, cooking food, digestion. |
Example:
Melting of ice → Physical (H₂O remains same).
Burning of candle → Both occur:
- Wax melting (physical)
- Wax burning to CO₂ + H₂O (chemical)
2.4 Types of Pure Substances
Pure substances are of two types:
- Elements
- Compounds
2.4.1 Elements
Definition:
A pure substance that cannot be broken down into simpler substances by chemical means.
Defined by:
- Robert Boyle (1661) — first used the term element.
- Antoine Lavoisier (1743–94) — gave first useful definition.
Types of Elements:
1. Metals
Properties:
- Shiny (lustrous)
- Silvery-grey or golden
- Good conductors of heat & electricity
- Ductile and malleable
- Sonorous (produce ringing sound)
Examples: Iron, copper, gold, aluminium, silver
Liquid metal: Mercury
2. Non-Metals
Properties:
- Dull appearance
- Poor conductors of heat & electricity
- Brittle
- Non-sonorous
Examples: Carbon, iodine, chlorine, oxygen, bromine (liquid non-metal)
3. Metalloids
Show properties of both metals and non-metals.
Examples: Boron, Silicon, Germanium.
2.4.2 Compounds
Definition:
- A substance formed when two or more elements chemically combine in a fixed ratio.
Examples:
Water (H₂O) → 2 parts hydrogen + 1 part oxygen
Carbon dioxide (CO₂) → 1 part carbon + 2 parts oxygen
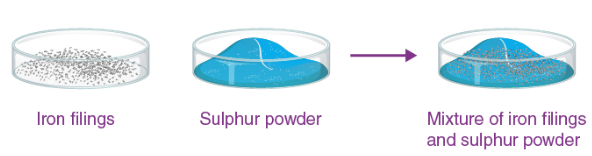
Iron + Sulphur Activity:
Mixture (Physical Change):
- Iron + sulphur mixed → can be separated with magnet → same properties retained.
Compound (Chemical Change):
- On heating → black solid (iron sulphide) → not magnetic → new properties formed.
Differences Between Mixtures and Compounds
| Mixtures | Compounds |
|---|---|
| Components just mix, no chemical reaction. | Elements react chemically to form new substance. |
| Variable composition. | Fixed composition. |
| Properties of components retained. | New properties formed. |
| Separated by physical methods. | Separated by chemical methods. |

Perfect notes