Notes For All Chapters Science Class 9 CBSE
1. Introduction
Atoms and molecules are the fundamental building blocks of matter.
The existence of different kinds of matter is due to different types of atoms.
Scientists questioned:
(i) What makes one atom different from another?
(ii) Are atoms indivisible as Dalton proposed?
Experiments showed that atoms are made up of sub-atomic particles – electrons, protons, and neutrons.
2. Charged Particles in Matter
Rubbing two objects together can produce electrical charges.
This indicates atoms contain charged particles.
J.J. Thomson (1897) discovered the electron – a negatively charged particle.
E. Goldstein (1886) discovered canal rays, which are positively charged → led to discovery of protons.
- Proton: positive charge (+1), mass = 1 unit.
- Electron: negative charge (–1), mass = negligible.
An atom contains protons and electrons, balancing each other’s charges.
3. Structure of the Atom
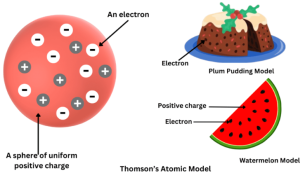
(a) Thomson’s Model (Plum Pudding Model)
Atom = a sphere of positive charge with electrons embedded in it (like seeds in watermelon).
Features:
- Atom has a positively charged sphere.
- Electrons are embedded in it.
- Total positive and negative charges are equal → atom is neutral.
Limitation: Could not explain later experimental results.
(b) Rutherford’s Model (Nuclear Model)
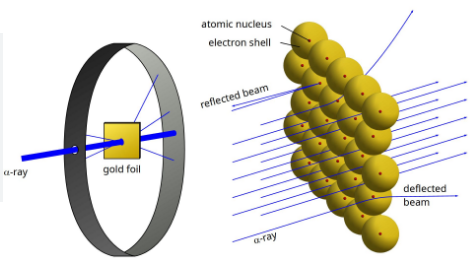
Conducted α-particle scattering experiment using thin gold foil.
Observations:
- Most α-particles passed straight.
- Some deflected at small angles.
- A few rebounded at 180°.
Conclusions:
- Most of atom is empty space.
- Positive charge occupies very little space.
- All positive charge and mass concentrated in nucleus.
Features of Rutherford’s model:
- Nucleus – small, dense, positively charged center; contains most of the mass.
- Electrons revolve around nucleus in circular paths.
- Size of nucleus is very small compared to the atom.
Drawback:
- Electrons revolving in orbits should lose energy and fall into nucleus → atom unstable (but atoms are stable).
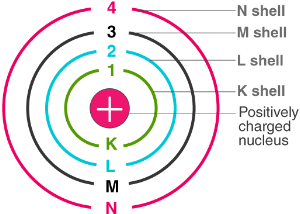
(c) Bohr’s Model
Neils Bohr improved Rutherford’s model (1913).
Postulates:
- Electrons revolve only in certain discrete orbits (energy levels).
- While revolving in these orbits, they do not radiate energy.
These orbits are called energy levels or shells: K, L, M, N (or n = 1, 2, 3, 4…).
(d) Neutrons
- J. Chadwick (1932) discovered neutron – neutral particle (no charge).
- Mass of neutron ≈ mass of proton.
- Neutrons are present in the nucleus (except in hydrogen).
- Mass of atom = protons + neutrons (called nucleons).
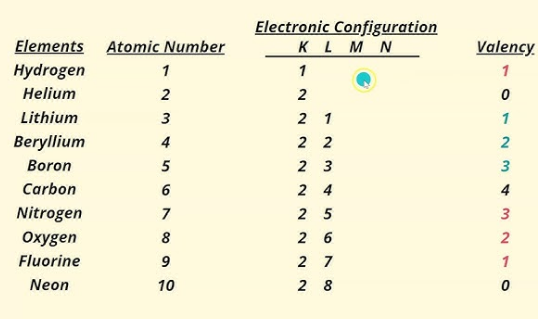
4. Distribution of Electrons in Orbits (Bohr–Bury Scheme)
Rules:
- Maximum electrons in a shell = 2n² (n = shell number).
- K = 2, L = 8, M = 18, N = 32.
- Outermost shell can have maximum 8 electrons.
- Inner shells must be filled first.
Example:
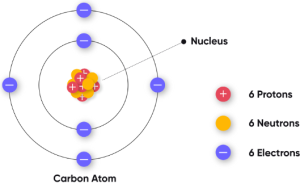
- Carbon (Z = 6): 2,4
- Sodium (Z = 11): 2,8,1
5. Valency
Valence electrons: electrons in the outermost shell.
Valency: combining capacity of an atom.
Atoms with full outer shells (like noble gases) are inert (valency = 0).
Atoms react to achieve a full outer shell (octet).
- By losing, gaining, or sharing electrons.
Examples:
- Hydrogen, lithium, sodium → lose 1 e⁻ → valency = 1.
- Magnesium → loses 2 e⁻ → valency = 2.
- Aluminium → loses 3 e⁻ → valency = 3.
- Fluorine → gains 1 e⁻ → valency = 1.
- Oxygen → gains 2 e⁻ → valency = 2.
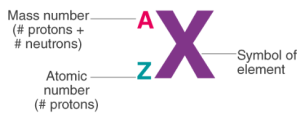
6. Atomic Number and Mass Number
Atomic Number (Z) = number of protons in nucleus.
- Defines the element.
- Example: H → Z = 1, C → Z = 6.
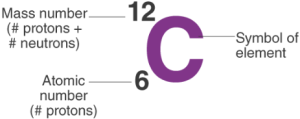
Mass Number (A) = number of protons + neutrons.
- Example: Carbon → 6 + 6 = 12.
Representation:
7. Isotopes
Atoms of the same element having same atomic number but different mass numbers.
Examples:
- Hydrogen → ¹H (Protium), ²H (Deuterium), ³H (Tritium).
- Carbon → ¹²C, ¹⁴C.
- Chlorine → ³⁵Cl, ³⁷Cl.
Properties:
- Same chemical properties.
- Different physical properties.
Average atomic mass = weighted mean of isotopic masses.
- Chlorine example: 35.5 u.
Uses of Isotopes:
- Uranium isotope → nuclear fuel.
- Cobalt isotope → cancer treatment.
- Iodine isotope → treatment of goitre.
8. Isobars
Atoms of different elements having same mass number but different atomic numbers.
Example:
Calcium (⁴⁰₂₀Ca) and Argon (⁴⁰₁₈Ar).
9. Key Points
Electron: discovered by J.J. Thomson (– charge).
Proton: discovered by E. Goldstein (+ charge).
Neutron: discovered by J. Chadwick (no charge).
Thomson’s Model – electrons embedded in positive sphere.
Rutherford’s Model – nucleus discovered; atom mostly empty.
Bohr’s Model – electrons revolve in fixed energy orbits.
Atomic Number (Z) = no. of protons.
Mass Number (A) = protons + neutrons.
Valency = combining capacity.
Isotopes = same Z, different A.
Isobars = same A, different Z.

Thanku