Solutions For All Chapters – Science Curiosity Class 7
Exploring Substances: Acidic, Basic, and Neutral
1. A solution turns the red litmus paper to blue. Excess addition of which of the following solution would reverse the change?
(i) Lime water
(ii) Baking soda
(iii) Vinegar
(iv) Common salt solution
Answer: (iii) Vinegar
2. You are provided with three unknown solutions labelled A, B, and C, but you do not know which of these are acidic, basic, or neutral. Upon adding a few drops of red litmus solution to solution A, it turns blue. When a few drops of turmeric solution are added to solution B, it turns red. Finally, after adding a few drops of red rose extract to solution C, it turns green.
Based on the observations, which of the following is the correct sequence for the nature of solutions A, B, and C?
(i) Acidic, acidic, and acidic
(ii) Neutral, basic, and basic
(iii) Basic, basic, and acidic
(iv) Basic, basic, and basic
Answer: (iv) Basic, basic, and basic
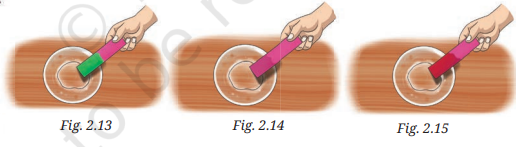
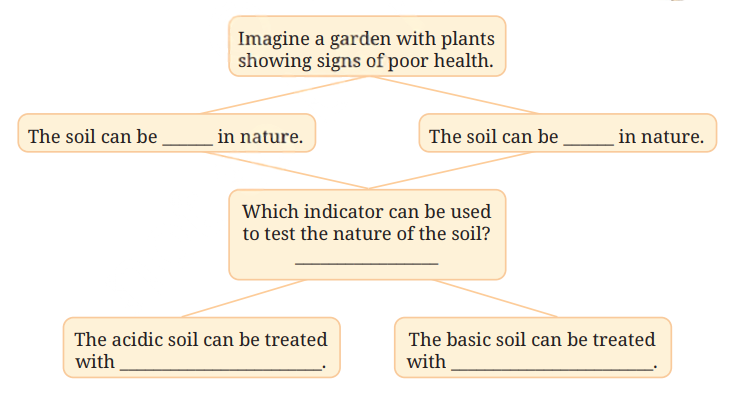
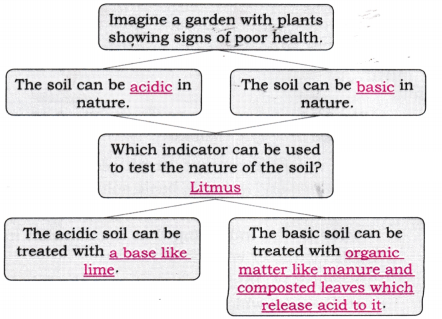
3. Observe and analyse Figs. 2.13, 2.14, and 2.15, in which red rose extract paper strips are used. Label the nature of solutions present in each of the containers.
Answer:
- Fig. 2.13: Basic (red rose extract turns green)
- Fig. 2.14: Neutral (red rose extract shows no change)
- Fig. 2.15: Acidic (red rose extract turns red)
4. A liquid sample from the laboratory was tested using various indicators:
Based on the tests, identify the acidic or basic nature of the liquid and justify your answer.
Answer: The liquid is acidic.
Justification: This is justified by the fact that blue litmus turned red, which indicates an acidic nature, while red litmus showed no change, consistent with its inability to change further in an acidic environment. Turmeric showed no change, which aligns with its property of not differentiating between acidic and neutral substances, further supporting the conclusion.
5. Manya is blindfolded. She is given two unknown solutions to test and determine whether they are acidic or basic. Which indicator should Manya use to test the solutions and why?
Answer: Manya should use an olfactory indicator like onion strips.
6. Could you suggest various materials which can be used for writing the message on the white sheet of paper (given at the beginning of the chapter) and what could be in the spray bottle? Make a table of various possible combinations and the colour of the writing obtained.
Answer: The colour of the writing will depend on the nature of the writing material (acidic or basic) and the solution in the spray bottle (acidic or basic). Below is the table of possible combinations.
| Material for Writing | Spray Bottle Content | Colour of Writing |
|---|---|---|
| Lemon juice | Blue litmus solution | Red |
| Baking soda solution | Red litmus solution | Blue |
| Baking soda solution | Rose extract | Green |
| Vinegar | Rose extract | Red |
| Soap solution | Turmeric solution | Red |
| Amla juice | Indian blackberry juice | Pink |
| Grapes juice | Beetroot extract | Red |
| Tamarind water | Red hibiscus extract | Pink |
7. Grape juice was mixed with red rose extract; the mixture got a tint of red colour. What will happen if baking soda is added to this mixture? Justify your answer.
Answer: If baking soda is added to the mixture of grape juice and red rose extract, the color is likely to change from a red tint to green.
Justification: This is because grape juice is acidic, which turns the red rose extract red, and baking soda is a basic substance. The red rose extract changes to green in the presence of a base, indicating a neutralization reaction where the acidic nature of the grape juice is altered by the basic baking soda.
8. Keerthi wrote a secret message to her grandmother on her birthday using orange juice. Can you assist her grandmother in revealing the message? Which indicator would you use to make it visible?
Answer: To help Keerthi’s grandmother reveal the secret message written with orange juice, she can use turmeric paper as an indicator. Orange juice is acidic, and while it doesn’t naturally change the color of turmeric paper (which remains yellow in acidic and neutral conditions), the message can be made visible by applying a basic substance, like baking soda solution, over the paper. When the basic solution interacts with the acidic orange juice in the message, the turmeric paper will turn red where the message is written, making it visible.
9. How can natural indicators be prepared? Explain by giving an example.
Answer: Natural indicators can be prepared by extracting color from certain flowers, fruits, or vegetables that change color in acidic or basic solutions. To prepare them, collect the material (like petals or juice), crush or boil it with water, and filter the liquid to get the indicator.
For example, to prepare a red rose extract indicator: Collect some fallen red rose petals, wash them, and crush them in a mortar. Place the crushed petals in a glass tumbler, pour hot water over them (with adult supervision), and let it sit until the water turns colored. Filter the liquid into a container; this filtrate is the red rose extract, which turns red in acidic solutions and green in basic solutions.
10. Three liquids are given to you. One is vinegar, another is a baking soda solution, and the third is a sugar solution. Can you identify them only using turmeric paper? Explain.
Answer: Yes, you can identify the liquids using only turmeric paper. Turmeric paper turns red in the presence of a basic substance but remains yellow in acidic and neutral substances. Vinegar is acidic, baking soda solution is basic, and sugar solution is neutral. When you test them, the turmeric paper will turn red with the baking soda solution, indicating it is basic. It will remain yellow with vinegar (acidic) and sugar solution (neutral), so you can distinguish vinegar and sugar solution from baking soda solution. However, to differentiate between vinegar and sugar solution, you’d need to note that no further change occurs with either, but this method alone confirms the basic liquid (baking soda) while leaving the other two indistinguishable without additional indicators.
11. The extract of red rose turns the liquid X to green. What will the nature of liquid X be? What will happen when excess of amla juice is added to liquid X?
Answer: The nature of liquid X is basic, as the red rose extract turns green in the presence of a basic substance. If excess amla juice, which is acidic, is added to liquid X, the green color of the red rose extract will likely change to red. This happens because the acidic amla juice neutralizes the basic nature of liquid X, causing the indicator to shift to its acidic color.
12. Observe and analyse the information given in the following flowchart. Complete the missing information.
Answer:


This is the best answer. It will be very helpful for me
It is very nice to take answers
Very nice solution I want solution for class 7 science all chapters
any questions ask me
thank you
very nice solutions. I want solutions for class 7th science all chapters.